read about
The Low Institute for Therapeutics (LIFT) is a non-profit drug discovery institute dedicated to reducing human suffering and mortality through research and drug discovery. Below is a list of publications that support our mission.
.png)
November 12, 2024
Fibroblast activation protein (FAP) has attracted considerable attention as a possible target for the radiotherapy of solid tumors. Unfortunately, initial efforts to treat solid tumors with FAP-targeted radionuclides have yielded only modest clinical responses, suggesting that further improvements in the molecular design of FAP-targeted radiopharmaceutical therapies (RPT) are warranted. In this study, we report several advances on the previously described FAP6 radioligand that increase tumor retention and accelerate healthy tissue clearance.
Read more >
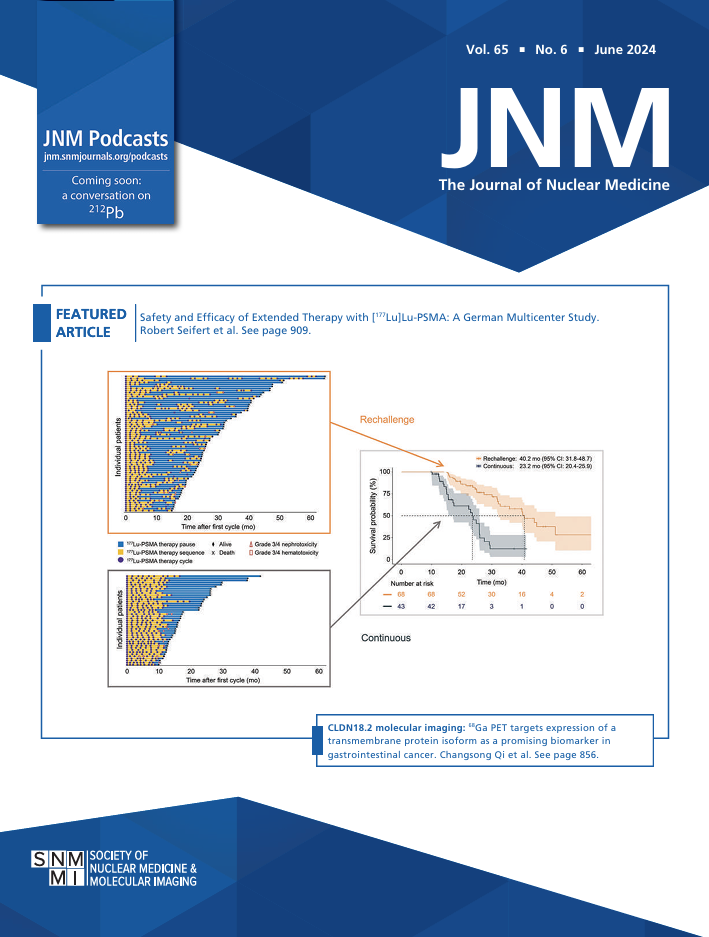
August 1, 2024
Because of upregulated expression on cancer-associated fibroblasts, fibroblast activation protein (FAP) has emerged as an attractive biomarker for the imaging and therapy of solid tumors. Although many FAP ligands have already been developed for radiopharmaceutical therapies (RPTs), most suffer from inadequate tumor uptake, insufficient tumor residence times, or off-target accumulation in healthy tissues, suggesting a need for further improvements. Methods: A new FAP-targeted RPT with a novel ligand (FAP8-PEG3-IP-DOTA) was designed by combining the desirable features of several previous ligand-targeted RPTs.
Read more >

July 25, 2024
Fibroblast activation protein (FAP) has attracted considerable attention as a possible target for the radiotherapy of solid tumors. Unfortunately, initial efforts to treat solid tumors with FAP-targeted radionuclides have yielded only modest clinical responses, suggesting that further improvements in the molecular design of FAP-targeted radiopharmaceutical therapies (RPT) are warranted. In this study, we report several advances on the previously described FAP6 radioligand that increase tumor retention and accelerate healthy tissue clearance.
Read more >

March 8, 2024
Spinal fusions are performed to treat congenital skeletal malformations, spondylosis, degenerative disk diseases, and other pathologies of the vertebrae that can be resolved by reducing motion between neighboring vertebrae. Unfortunately, up to 100,000 fusion procedures fail per year in the United States, suggesting that efforts to develop new approaches to improve spinal fusions are justified. We have explored whether the use of an osteotropic oligopeptide to target an attached bone anabolic agent to the fusion site might be exploited to both accelerate the mineralization process and improve the overall success rate of spinal fusions.
Read more >
.png)
February 7, 2024
Folate receptors can perform folate transport, cell adhesion, and/or transcription factor functions. The beta isoform of the folate receptor (FRβ) has attracted considerable attention as a biomarker for immunosuppressive macrophages and myeloid-derived suppressor cells, however, its role in immunosuppression remains uncharacterized. We demonstrate here that FRβ cannot bind folate on healthy tissue macrophages, but does bind folate after macrophage incubation in anti-inflammatory cytokines or cancer cell-conditioned media.
Read more >
.png)
October 20, 2023
Folate receptor delta (FRδ) has been used as a biomarker for regulatory T cells (Tregs), because its expression is limited to Tregs and ovum. Although FRδ is unable to bind folate, we have used molecular docking software to identify a folate congener that binds FRδ with high affinity and have exploited this FRδ-specific ligand to target attached drugs (imaging agents, immune activators, and immune suppressors) specifically to Tregs in murine tumor xenografts. Analysis of treated tumors demonstrates that targeting of a Toll-like receptor 7 agonist inhibits Treg expression of FOXP3, PD-1, CTLA4, and HELIOS.
Read more >
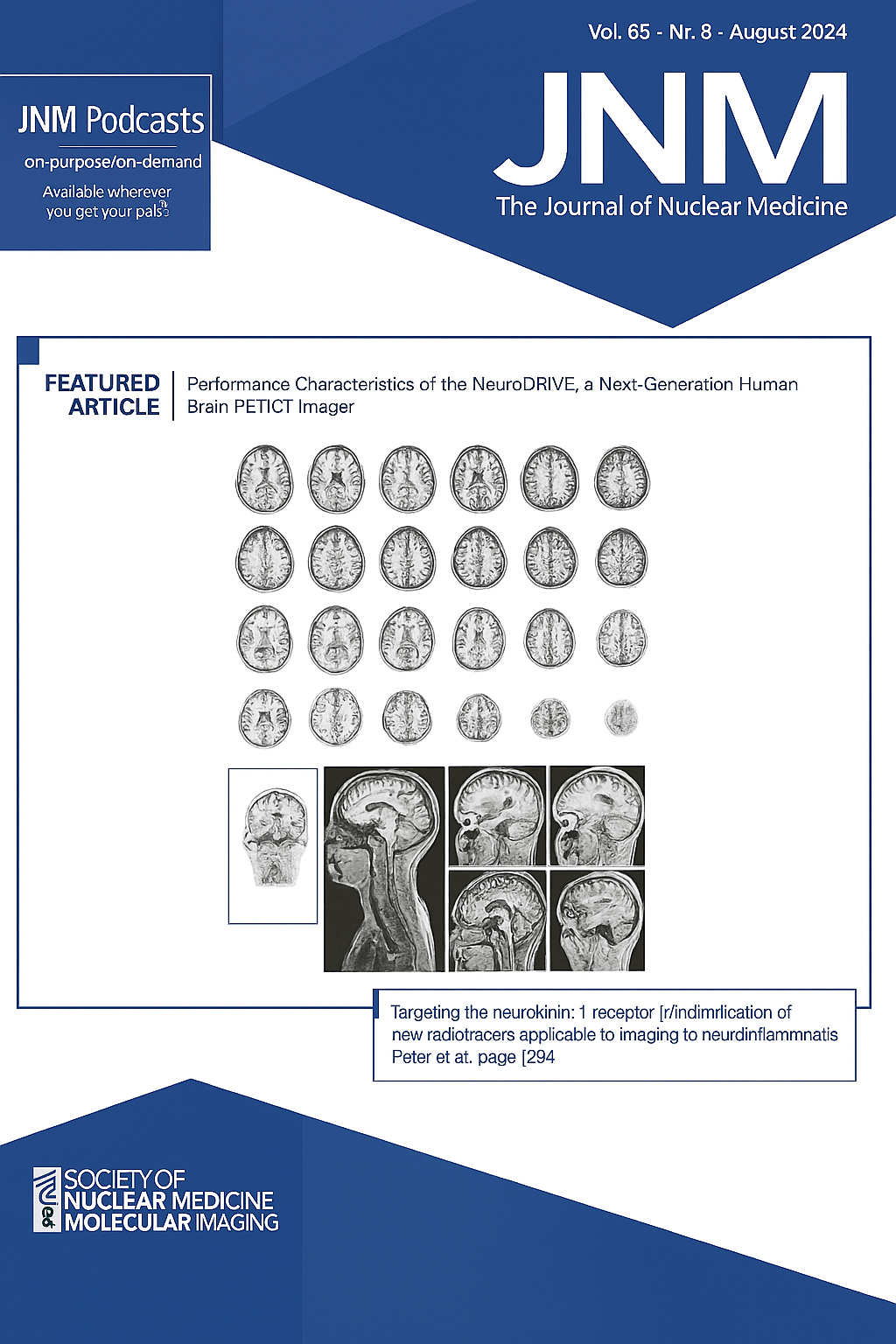
May 1, 2023
Fibroblast activation protein (FAP) has received increasing attention as an oncologic target because of its prominent expression in solid tumors but virtual absence from healthy tissues. Most radioligand therapies (RLTs) targeting FAP, however, suffer from inadequate tumor retention or clearance from healthy tissues. Herein we report a FAP-targeted RLT comprising an FAP6 ligand conjugated to DOTA and an albumin binder (4-p-iodophenylbutyric acid, or IP) for enhanced pharmacokinetics. We evaluated the performance of the resulting FAP6-IP-DOTA conjugate in 4 tumor models, 3 of which express FAP only on cancer-associated fibroblasts, that is, analogously to human tumors.
Read more >

September 7, 2022
Targeted drug delivery, often referred to as “smart” drug delivery, is a process whereby a therapeutic drug is delivered to specific parts of the body in a manner that increases its concentration at the desired sites relative to others. This approach is poised to revolutionize medicine as exemplified by the recent FDA approval of Cytalux (FDA approves pioneering drug for ovarian cancer surgery - Purdue University News) which is a folate-receptor targeted intraoperative near infrared (NIR) imaging agent that was developed in our laboratories. Fracture-associated morbidities and mortality affect a significant portion of world population.
Read more >

March 7, 2022
Folate receptor beta (FR-β) has been used as a clinical marker and target in multiple inflammatory diseases, including osteoarthritis (OA) and rheumatoid arthritis (RA). However, the conditions under which FR-β+ macrophages arise remain unclear and could be affected by corticosteroids. Therefore, we studied FR-β expression in vitro in macrophage subtypes and determined their response to triamcinolone acetonide (TA), a clinically often-used corticosteroid. Human monocyte–derived macrophages were differentiated to the known M0, M1, or M2 macrophage phenotypes.
Read more >

February 22, 2022
Tumor-targeted fluorescent dyes have been shown to significantly improve a surgeon's ability to locate and resect occult malignant lesions, thereby enhancing a patient's chances of long term survival. Although several tumor-targeted fluorescent dyes have been developed for imaging specific subsets of human cancers, no tumor-targeted dye has been designed that can image all cancer types. Based on observations that fibroblast activation protein (FAP) is upregulated on cancer-associated fibroblasts (CAFs) that infiltrate essentially all solid tumors, we have undertaken to develop a FAP-targeted fluorescent dye that can image CAFs without accumulating in healthy cells or fibroblasts.
Read more >
.png)
February 1, 2022
Non-invasive imaging modalities constitute an increasingly important tool in diagnostic and therapy response monitoring of patients with autoimmune diseases, including rheumatoid arthritis (RA). In particular, macrophage imaging with positron emission tomography (PET) using novel radiotracers based on differential expression of plasma membrane proteins and functioning of cellular processes may be suited for this. Over the past decade, selective expression of folate receptor β (FRβ), a glycosylphosphatidylinositol-anchored plasma membrane protein, on myeloid cells has emerged as an attractive target for macrophage imaging by exploiting the high binding affinity of folate-based PET tracers.
Read more >

October 4, 2021
To egress from its erythrocyte host, the malaria parasite, Plasmodium falciparum, must destabilize the erythrocyte membrane by activating an erythrocyte tyrosine kinase. Because imatinib inhibits erythrocyte tyrosine kinases and because imatinib has a good safety profile, we elected to determine whether coadministration of imatinib with standard of care (SOC) might be both well tolerated and therapeutically efficacious in malaria patients.
Read more >

February 1, 2021
This Cancer Research study shows that folate receptor-β (FRβ) defines an immunosuppressive subset of tumor-associated myeloid cells—MDSCs and macrophages—across mouse and human tumors. Because FRβ is selectively expressed on these cells, folate-linked drugs can be delivered directly to them. Targeted stimulation (e.g., a folate-conjugated TLR7 agonist) reprogrammed these cells, reduced suppressive activity, increased CD8+ T-cell infiltration, shifted macrophages toward pro-inflammatory phenotypes, and slowed tumor growth in mice. FRβ is thus both a marker and a therapeutic entry point to remodel the tumor microenvironment.
Read more >

January 10, 2021
This Journal of Controlled Release study evaluates a bone fracture–targeting platform that systemically delivers pro-healing agents directly to the injury. Angiogenic peptides (e.g., the VEGF-mimetic QK) are conjugated to acidic oligopeptides that bind exposed hydroxyapatite, concentrating drug at the fracture callus while sparing healthy tissues. In murine femur-fracture models—including diabetic mice—the targeted therapy increased callus BV/TV and maximum load versus saline and showed no adverse liver or kidney effects. The approach aims to accelerate revascularization and repair by boosting local efficacy and reducing systemic exposure.
Read more >

October 28, 2020
Idiopathic pulmonary fibrosis (IPF) is driven by collagen-producing myofibroblasts. This study targets those cells via fibroblast activation protein (FAP), using a small-molecule ligand to deliver a PI3K/mTOR inhibitor to profibrotic fibroblasts. In patient-derived cells, the conjugate suppressed PI3K signaling and collagen synthesis. In a mouse model, systemic dosing focused activity in diseased lungs, lowered hydroxyproline and histologic fibrosis, and improved survival. By limiting drug action to FAP-positive cells, the approach supports a selective, disease-modifying strategy for IPF.
Read more >

August 29, 2020
Acidic oligopeptides have the ideal half-life, toxicity profile, and selectivity for a bone fracture-targeting ligand and are the most developed and promising of these bone fracture-targeting ligands. However, many other promising ligands have been developed that could be used for bone fractures.
Read more >
August 14, 2020
Although artemisinin-based combination therapies (ACTs) treat Plasmodium falciparum malaria effectively throughout most of the world, the recent expansion of ACT-resistant strains in some countries of the Greater Mekong Subregion (GMS) further increased the interest in improving the effectiveness of treatment and counteracting resistance.
Read more >

June 29, 2020
Fibrotic diseases cause organ failure that lead to ~45% of all deaths in the United States. Activated macrophages stimulate fibrosis by secreting cytokines that induce fibroblasts to synthesize collagen and extracellular matrix proteins. Although suppression of macrophage‐derived cytokine production can halt progression of fibrosis, therapeutic agents that prevent release of these cytokines (e.g., TLR7 agonists) have proven too toxic to administer systemically.
Read more >
April 27, 2020
Cancer-associated fibroblasts (CAFs) comprise a major cell type in the tumor microenvironment where they support tumor growth and survival by producing extracellular matrix, secreting immunosuppressive cytokines, releasing growth factors, and facilitating metastases. Because tumors with elevated CAFs are characterized by poorer prognosis, considerable effort is focused on developing methods to quantitate, suppress and/or eliminate CAFs. We exploit the elevated expression of fibroblast activation protein (FAP) on CAFs to target imaging and therapeutic agents selectively to these fibroblasts in solid tumors.
Read more >

June 7, 2019
Most therapies for autoimmune and inflammatory diseases either neutralize or suppress production of inflammatory cytokines produced by activated macrophages (e.g., TNFα, IL-1, IL-6, IL-17, GM-CSF). However, no approved therapies directly target this activated subset of macrophages.
Read more >

October 31, 2018
Approximately 6.3 million bone fractures occur annually in the United States, resulting in considerable morbidity, deterioration in quality of life, loss of productivity and wages, and sometimes death (e.g., hip fractures). Although anabolic and antiresorptive agents have been introduced for treatment of osteoporosis, no systemically administered drug has been developed to accelerate the fracture-healing process. To address this need, we have undertaken to target a bone anabolic agent selectively to fracture surfaces in order to concentrate the drug’s healing power directly on the fracture site.
Read more >

September 22, 2015
To evaluate the fracture healing capabilities of a GSK3β inhibitor, 6-bromoindirubin-3′-oxime, coupled with an aspartic acid octapeptide in a micellar delivery system. Materials & methods: The efficacy of the intravenously administered micelles to accelerate healing of femoral fracture in mice was evaluated. Micro-computed tomography analysis was employed to obtain bone density, total volume, relative volume, trabecular thickness and trabecular spacing.
Read more >

September 2, 2015
Bone fractures constitute a major cause of morbidity and mortality especially in the elderly. Complications associated with osteoporosis drugs and the age of the patient slow bone turnover and render such fractures difficult to heal. Increasing the speed of fracture repair by administration of a fracture-targeted bone anabolic agent could find considerable application. Aspartic acid oligopeptides are negatively charged molecules at physiological pH that adsorb to hydroxyapatite, the mineral portion of bone. This general adsorption is the strongest where bone turnover is highest or where hydroxyapatite is freshly exposed. Importantly, both of these conditions are prominent at fracture sites.
Read more >

Low Institute For Therapeutics
3000 Kent Ave, Suite 1956
West Lafayette, IN 47906

Low Institute For Therapeutics
3000 Kent Ave, Suite 1956
West Lafayette, IN 47906